Jemema Agnes Tripena Raj
India
Computational Identification of Natural Dual PPARα/γ Modulators as Neuroprotective Candidates Against Radiation-Induced Cognitive Decline
Jemema Agnes Tripena Raj¹˒²,Shubham Ghanekar¹, Isha Shinde¹˒², Abhishek Chatterjee¹˒²,
Nikhil Gadewal¹˒², Pratik Chandrani¹˒², Jayant S. Goda¹˒²
Affiliations
¹ Advanced Centre for Treatment, Research and Education in Cancer (ACTREC), Tata Memorial Centre, Mumbai, India
² Homi Bhabha National Institute (HBNI), Mumbai, India
Abstract
Background
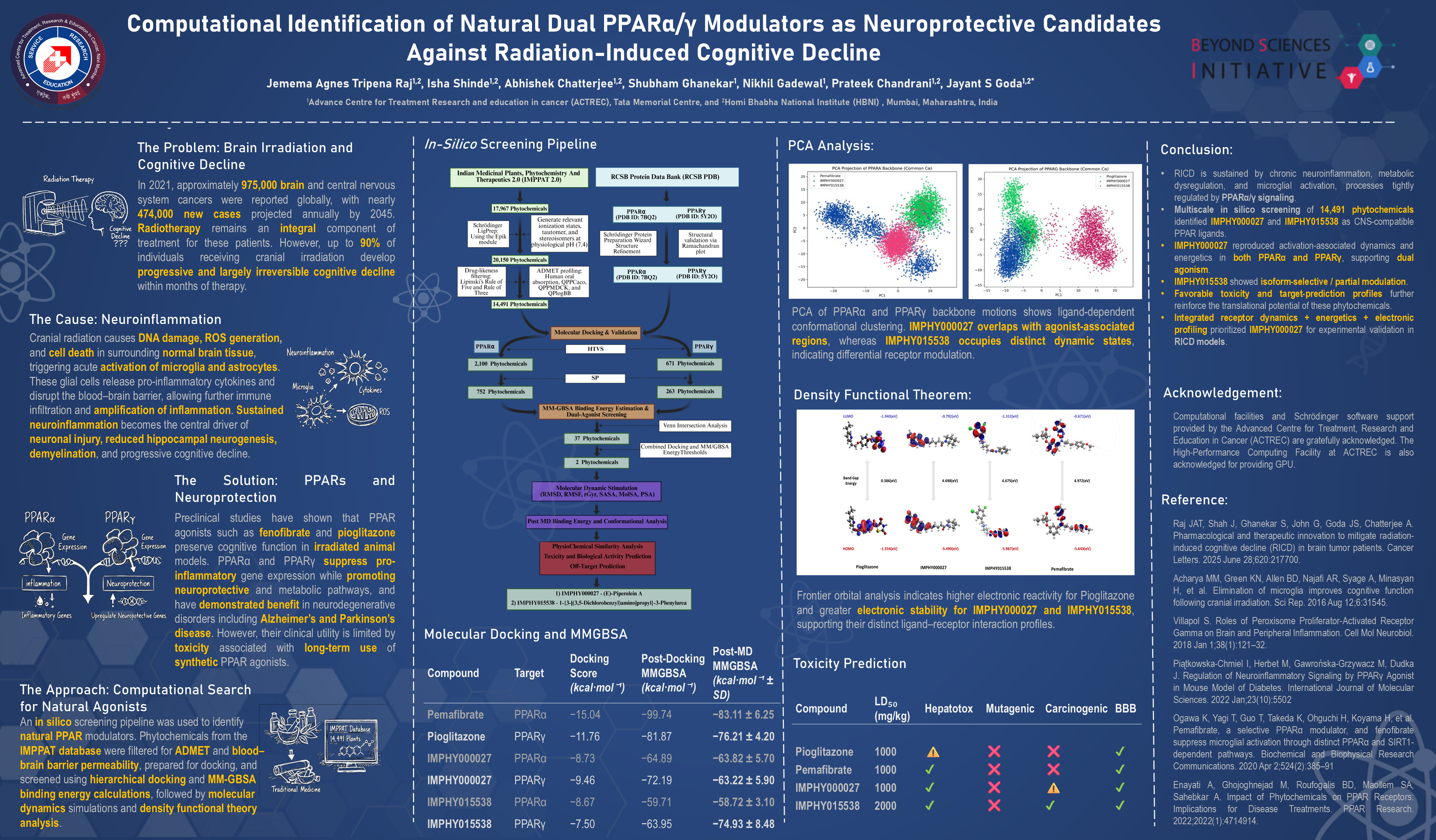
Radiation-induced cognitive decline (RICD) arises from chronic oxidative stress, neuroinflammation, and disrupted neurogenesis following cranial irradiation. Peroxisome Proliferator-Activated Receptors (PPARα/γ) regulate lipid metabolism, mitochondrial health, and inflammatory signaling and are emerging neuroprotective targets. This study aimed to identify natural phytochemicals with predicted dual PPARα/γ engagement using a multi-layered in silico approach integrating ligand screening, molecular dynamics, and receptor-motion analysis.
Methods
A total of 14,491 phytochemicals from the IMPPAT database were screened using hierarchical docking, MM-GBSA rescoring, ADMET profiling, and BBB-permeability filtering. Lead complexes were evaluated through 100-ns molecular dynamics (MD) simulations, followed by RMSD/RMSF stability assessment. Principal component analysis (PCA) and PC1–PC2 free-energy landscapes (FELs) were generated to quantify ligand-induced receptor motions. MM-GBSA–PCA correlations were computed to evaluate coupling between global receptor dynamics and predicted binding energetics.
Results
Two compounds—IMPHY000027 ((E)-piperolein A) and IMPHY015538—demonstrated favorable docking scores, stable MD trajectories, and binding energies comparable to Pemafibrate and Pioglitazone. IMPHY000027 consistently stabilized activation-associated conformations in both PPARα and PPARγ, showing strong occupancy of agonist-like PCA clusters and coherent low-energy basins. MM-GBSA correlation revealed meaningful coupling between PC1 motion and binding free energy, supporting its predicted dual-agonist behavior. In contrast, IMPHY015538 populated distinct PCA regions and fragmented FELs with minimal energetic coupling, suggesting isoform-selective or partial agonism. Toxicity and activity predictions indicated lower predicted toxicities and plausible anti-inflammatory properties relative to synthetic reference agonists.
Conclusions
The integrated docking–MD–PCA–FEL workflow identified IMPHY000027 as a promising natural dual PPARα/γ modulator with mechanistic support from conformational dynamics and binding-energy correlations. IMPHY015538 showed receptor-specific behavior compatible with selective or partial agonism. These findings provide a computational basis for prioritizing phytochemicals for experimental evaluation in the context of neuroinflammation and radiation-induced cognitive decline.

Leave A Comment