Neveen Salem
Egypt
Synthetic antiprotozoal thiazolide drug induced apoptosis in colorectal cancer cells
Mohamed A. Tantawy1, Nagla A. El-Sherbeeny2, Nawal Helmi3, Reem Alazragi3, Neveen Salem4 & Samah M. Elaidy2
1.Hormones Department, Medical Research Division, National Research Centre, Dokki, Giza, 12622, Egypt
2.Department of Clinical Pharmacology, Faculty of Medicine, Suez Canal University, Ismailia, 41522, Egypt
3.Department of Biochemistry, College of Science, University of Jeddah, Jeddah, Saudi Arabia
4.Narcotics, Ergogenic Aids and Poisons Department, National Research Centre, Dokki, Giza, 12622, Egypt
Abstract
Background
Colorectal cancer (CRC) is a global pressing healthcare priority. Dysregulation of the IL6/JAK2/STAT3 and p53/caspase
downstreaming pathways are significantly involved in the progression of CRC, and mainly affecting apoptosis. Discovery of
new anti-cancer agents is laborious, time consuming, and costly with obvious socioeconomic burden
Methods
In the present study,
we are proposing new molecular insights on the anti-proliferative and apoptotic therapeutic effects of nitazoxanide (NTZ)
on CRC. NTZ is FDA-approved thiazolide antiparasitic agent, which has excellent safety and pharmacokinetic profiles
Results
The
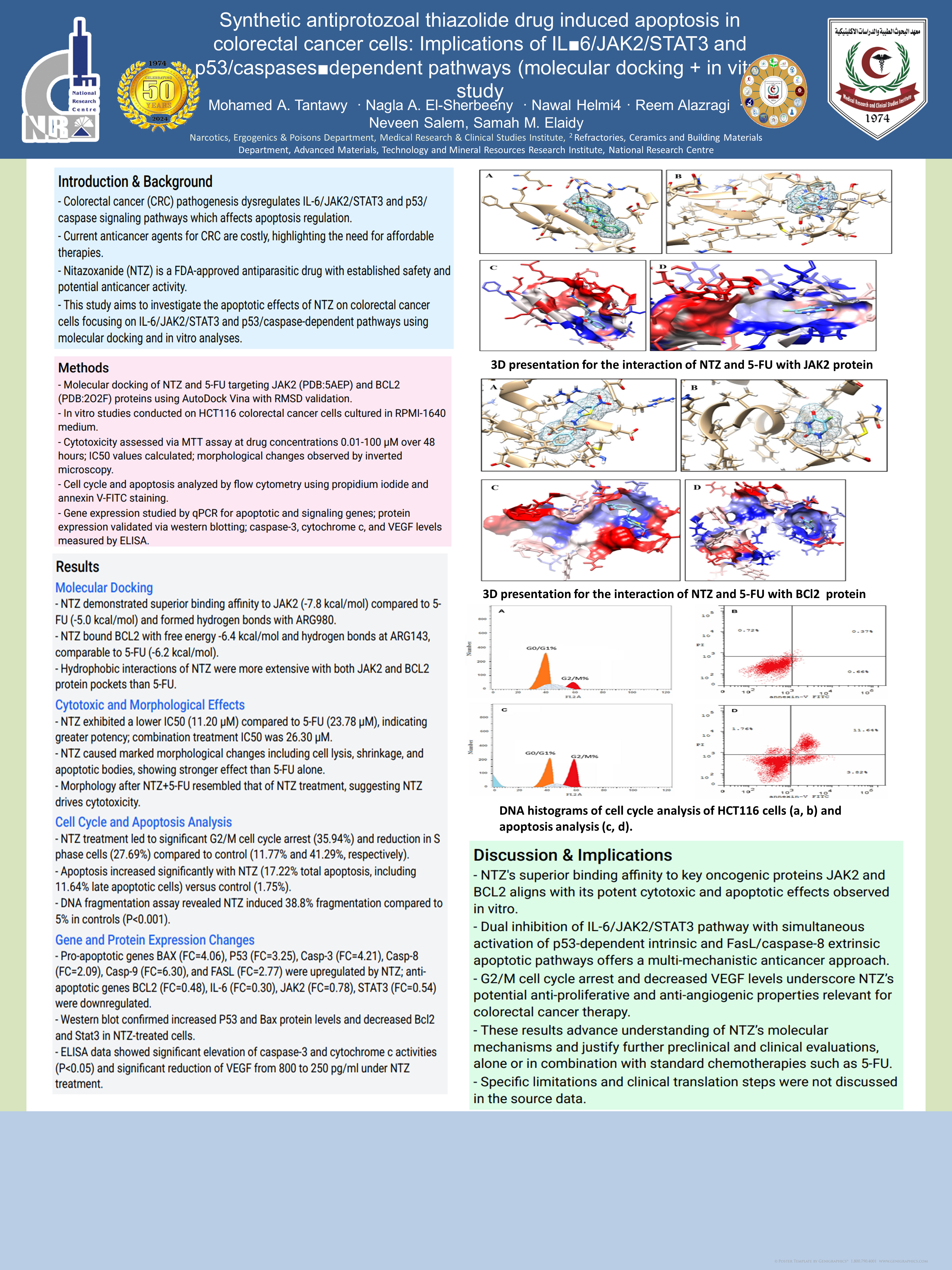
molecular docking study revealed that NTZ has better binding affinity and docking score against JAK2 and BCL2 proteins
compared to 5-Fluorouracil, which is the standard drug for treatment of CRC. The current in vitro work on a human HCT116
cell line displayed that NTZ had lower IC50 value (11.20 µM) than 5-flurouracil (23.78 µM), and NTZ induced a statistically
significant down-regulation of IL6/JAK2/STAT3. NTZ also modulated significantly the p53/caspases-dependent signaling pathways, leading to enhancement of apoptosis and an increase of DNA fragmentation. Moreover, NTZ regulated the
Bcl-2 gene family and promoted the loss of mitochondrial function which was depicted by release of cytochrome c (Cyt c),
and caspase activation in apoptotic HCT116 cells. Additionally, NTZ was able to reduce the expression of VEGF in CRC
cell line, which needs future thorough molecular investigations.
Conclusions
our findings provided a novel evidence that
NTZ could be a dual potential IL6/JAK2/STAT3 signaling inhibitor and p53/caspases-dependent pathway activator in CRC
cell line. These potentials support further exploratory molecular researches targeting the therapeutic roles of NTZ in CRC;
individually and simultaneously with current approved chemotherapeutic regimens.


Leave A Comment