Conference 2021 Poster Presentation
Project title
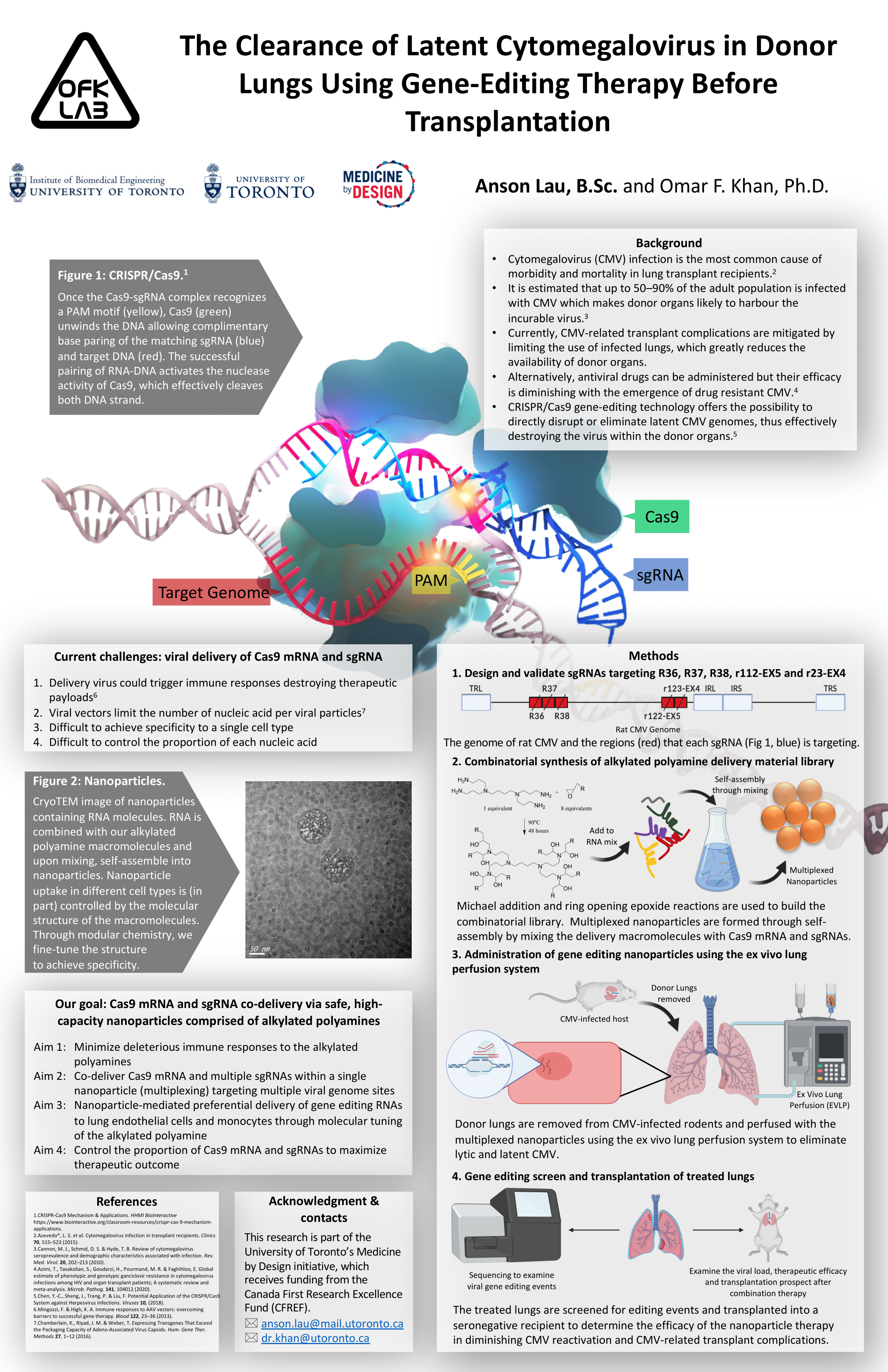
The Clearance of Latent Cytomegalovirus in Donor Lungs Using Gene-Editing Therapy Before Transplantation.
Authors and Affiliations
Anson Lau1, Omar F. Khan1,2
1. Institute of Biomedical Engineering, University of Toronto, Toronto, Canada
2. Department of Immunology, University of Toronto, Toronto, Canada
Abstract
Background
Cytomegalovirus (CMV) infection is among the most common cause of morbidity and mortality in lung transplant recipients. It is estimated that up to 50–90% of the adult population is infected by CMV, making donor organs likely to harbour the incurable virus. Gene editing technology where CRISPR/Cas9 cleaves regions of viral DNA offers a promising solution to eliminate CMV in the donor lungs. Traditionally, gene-editing payloads are delivered virally. However, viral delivery could risk permanent alteration of the host genome, trigger inflammatory reactions and has a relatively small payload capacity. Contrastingly, we hypothesize that our synthetic, high-capacity nanoparticles can transiently co-deliver a controlled dosage of multiple viral gene-targeting single guide RNAs (sgRNAs) and Cas9 mRNA (multiplexing) to eliminate latent CMV in infected donor lungs without inducing immunogenic responses.
Methods
SgRNAs are designed to target 5 immediate-early (IE) genes of CMV, they are R36, R37, R38, r122-EX5 and r123-EX4. After, nanoparticles are made using michael addition and ring open epoxide reaction and subsequently mixed with the sgRNAs and Cas9 mRNA to self-assemble into the multiplexed nanoparticles. In parallel, LEWIS rats are latently infected with rat CMV and their lungs are removed and perfused with the multiplexed nanoparticles using ex vivo lung perfusion (EVLP). The treated lungs are then screened for cytotoxicity and editing events and immediately transplanted into a seronegative recipient to determine the transplantation prospect and therapeutic efficacy.
Results
(Expected) The delivery of CRISPR/Cas9 nucleic acids using our nanoparticles in EVLP is expected to destroy latent CMV genomes residing in the lungs with minimal immunogenic response and cytotoxicity. Viral genome sequencing is expected to observe indels at the 5 targeted IE genes site. The viral load upon reactivation within transplanted recipients is expected to decrease. Due to the decrease in viral load, CMV-related lung transplant complications are expected to decrease along with an increase in life expectancy.
Conclusions
By eliminating latent CMV within donor lungs before transplantation, the pool of viable donor lungs can be drastically increased to meet the growing need for donor organs. Furthermore, CMV-related transplant complications can also be greatly reduced which can improve the transplantation prospect and recipient life-expectancy.